Polymers & Composites
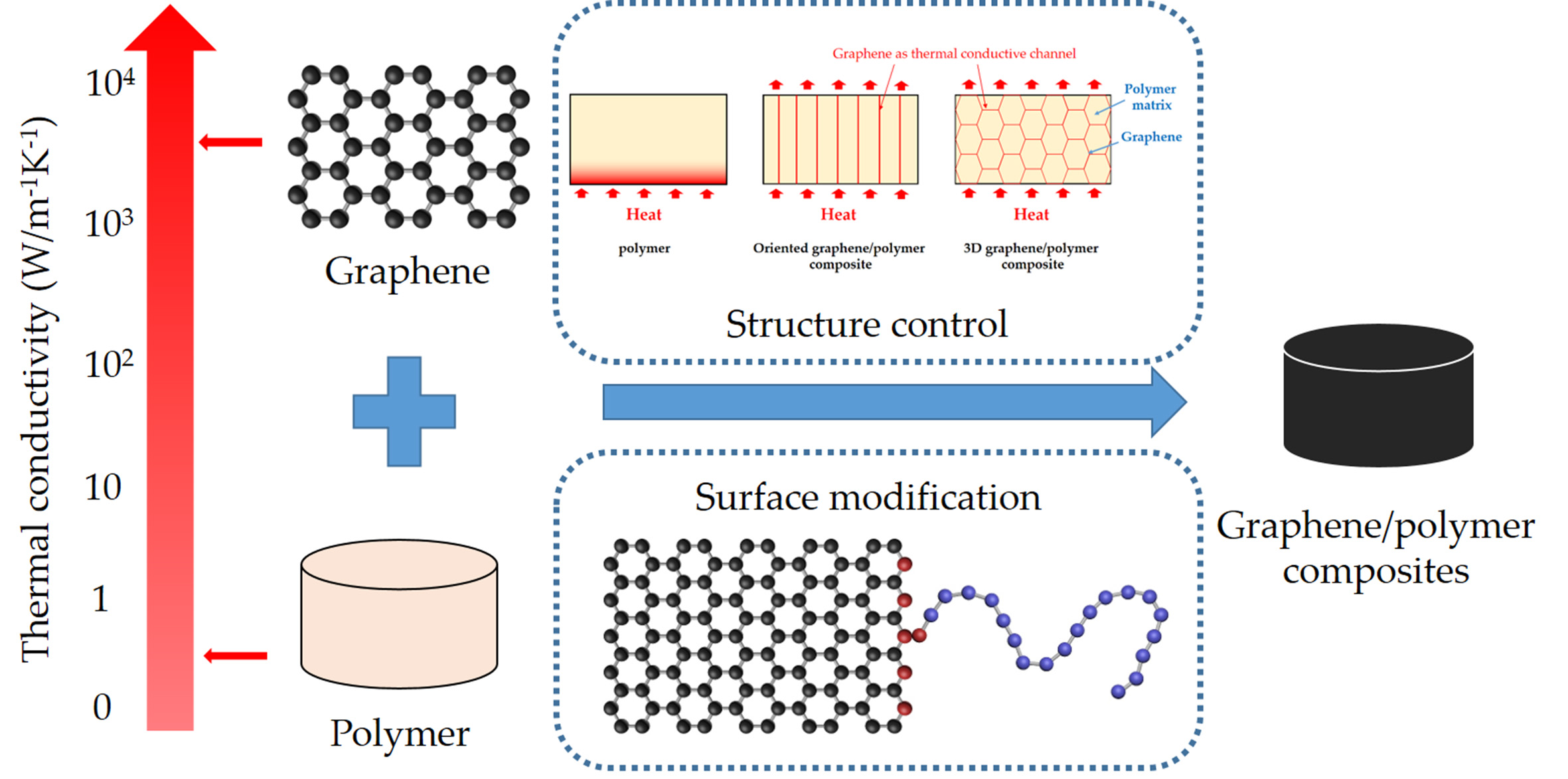
-a) Definition
–monomer, polymer, degree of freedom, functionality, oligo polymer, High
polymer, isotactic, atactic, syndiotactic, Homopolymers, Heteropolymers.
b) Types of
polymerization – Addition, condensation , co-polymerisation – comparison
c)Mechanism of Free radical polymerization – 3 step process
c)Mechanism of Free radical polymerization – 3 step process
Step1-Initiation
(Radical and Chain initiating species formation)
Step2 -Propagation (Living polymer formation)
Step3 -Termination
(By coupling and disproportionation)
2. Plastics
a) Classification
– i)based on thermal property – Thermo / Thermoset plastics ii) Based on usage – Commodity and
Engineering plastics
b) Prepration,
properties and applications of i)
PVC ii) Teflon
iii) Poly carbonate iv) Poly
Urethane v)PET vi) Nylon
3. Rubber
a) Raw rubber –
Preparation - problems of raw rubber – Vulcanisation – Difference between raw
and vulcanized rubber
b)Synthetic rubber
– Preparation, properties, uses of i) SBR
ii) Butyl rubber
4. Composites
a) Definition – properties - Types -
Polymer matrix / Metal matrix / Ceramic matrix composties
b) Fibre
Reinforced Plastics (FRP) – Types of FRP
- Applications of FRP
TOPIC -1. POLYMERISATION:
1. Under the proper conditions of temperature, pressure and
catalyst , the micro (Smaller) molecules are combining together to form a macro
(big) molecule. This process is called Polymerisation.
E.g n(CH2 = CH2 ) à --(CH2 - CH2 )—n
2. Micro molecules are called ‘Monomer’. Macro molecule is ‘Polymer’.
Monomer is a micro
molecule (small molecule) which combines with each other to form a polymer.
Example: ethylene.
Condition for a
monomer:
b. It
should contain minimum two same or different functional groups.
3. The number of monomers present in a polymer is ‘ Degree
of polymerisation’ (n).
Degree of Polymerisation = Molecular Wt of polymer / Molecular Wt
of monomer
n=
M/m
If n = low , Mol.Wt = 500 – 5000 Dalton units, it is Oligo
polymer.
If n = High, Mol.Wt = 10,000 –
2,00,000 Dalton units , it is High polymer.
4. If the polymer chain contains same type of monomer, it is
“Homo polymer”.
e.g PVC
structure : A – A – A- A- A-A -A
If the polymer
chain contains different type of monomer, it is “Hetero polymer” or
Copolymer.
e.g. Nylon A-B-
A-A-A-B-A
Types of Copolymers:
i)
Alternating copolymer: M1-M2-M1-M2-M1-M2
ii)
Random copolymer: M1-M2-M2-M1-M2-M1
iii)
Block
copolymer: M1-M1-M1-M2-M2-M2-
M1-M1-M1-M2-M2-M2
iv)
Graft copolymer: M1-M1-M1-M1-M1-M1
l
M2
l
M2
5. Number of Reactive sites present in a monomer is called ‘Functionality’.
e.g CH2 = CH2 , The double bond is acting as two reactive
site, So, Ethylene functionality is 2.
CH2 – OH In glycerol three –OH groups present.
So, functionality = 3
│
CH – OH
│
CH2 – OH
Significances:
1. If
F = 2, they form linear chain structure.
2. If
F=3, they form branched structure.
3. If
F≥ 4, then they form complex 3D structure.
6. Orientation of monomers in a polymer chain is called
“Tacticity”. If the groups are in same
orientation, it is isotactic. If they
are random it is “atactic”. If they are
arranged in alternative fashion, it is syndiotactic.
A A
A A A
A B B
A B A B
A B A
│ │
│ │ │ │ │
│ │ │ │ │
│ │ │
M
-M - M
- M -
M - M - M
- M
- M –M M
- M - M – M - M
│
│ │ │
│ │ │
│ │ │
│ │ │
│ │
B B
B B B
B A A
B A B A
B A B
(Iso tactic) (Atactic) (syndiotactic)
Cis polyisoprene polypropylene Trans
polyisoprene
TYPES OF POLYMERISATION :
1. Addition 2.
Condensation 3. Copolymerisation
No
|
Addition Polymerisation
|
Condensation Polymerisation
|
1
|
Eg. PVC
|
Eg. Nylon 6,6
|
2
|
Otherwise known as “Chain growth Polymerisation”.
|
Otherwise known as “Step wise Polymerisation”.
|
3
|
Monomers are adding together to form polymers.
|
Monomers are condensed to form polymer.
|
4
|
No elimination of other molecules occurs.
|
Elimination of smaller molecules occurs.
|
5
|
At least one multiple bond presence is essential
condition.
|
Monomers must have two or more functional groups.
|
6
|
Homo polymers are formed.
|
Hetero polymers are formed.
|
7
|
Thermoplastics are formed.
|
Thermo set plastics are formed.
|
8
|
Molecular weight of the polymer is the integral multiple
of monomers.
|
Need not be so.
|
9
|
Monomers disappear slow and steadily.
|
Monomers disappear at the initial stage of the reaction.
|
10
|
Longer processing time is needed.
|
Longer time is essential.
|
e.g
Addition
Polymerisation:
n(CH2 = CH2
) à -( CH2 - CH2 -)n
Ethylene à
Polyethylene
Condensation
Polymerisation
n H2N - (CH2)6 – NH2 + n
HOOC – (CH2)4 – COOH
à
Hexa methylene diamine Adipic
acid
[ - HN - (CH2)6 – NH - OC –
(CH2)4 – CO - ]n
Nylon 6,6
Co-Polymerisation
1. It is a special kind of polymerisation, otherwise known
as “Joint polymerisation”.
The product is known as ‘Co-polymers’. It is used to alter the hardness, strength,
rigidity of the monomers.
n CH2 = CH - CH
= CH2 + n à
( 75% butadiene)
(25% Styrene)
--[ CH2 - CH = CH
- CH2 - CH2 - CH
-]n
(Styrene
– Butadiene RubberSBR)
MECHANISM OF FREE RADICAL ADDITION POLYMERISATION :
3 steps in Free
radical mechanism: 1. Initiation 2.
Propagation 3. Termination
Step I - Initiation : 1a) Initiator
à Radical
1b)
Radical + Monomer
à Chain Initiating Species (CIS)
Step II - Propagation: CIS
+ n (monomer) à Living polymer
Step III - Termination:
3a) By Coupling
: Radical + Radical
à Macromolecule
( Dead polymer)
3b) By
disproportionation (by Hydrogen transformation):
Radical +
Radical à Unsaturated polymer + Saturated polymer
EXPLANATION:
1. Initiation
a) Initiator
à Radical
1.The substance which undergoes homolytic cleavage to form
radical is called ‘Initiator’. (e.g)
acetyl peroxide initiator
2.The substance with single electron is called ‘ radical’.
(e.g) acetyl peroxide radical
e.g Acetyl
peroxide à Radicals
( at 800C) CH3COO - CH3COO à 2 CH3COO.
(or)R.
b) Radical
+ Monomer à Chain Initiating Species (CIS)
H H
│ │
R. + CH2=C à R – CH2 - C.
│
│
Cl
Cl
2. Propagation:
CIS + n
(monomer) à Living polymer
H
H H H
│
│ │ │
R – CH2 - C∙ +
n ( CH2 =
C ) à R (-CH2 – C -)n-CH2
- C.
│
│ │ │
Cl Cl Cl Cl
3. Termination
a) Coupling
Radical + Radical
à Macromolecule
( Dead polymer)
CIS + CISà Dead polymer
H H H H
│ │ │ │
R – CH2 - C∙ +
R- CH2 – C. à R – CH2 – C – C – CH2
– R
│
│ │
│
Cl Cl
Cl Cl
(Dead polymer)
b) Disproportionation (by Hydrogen transformation)
CIS + CIS à Unsaturated
polymer + Saturated polymer
H H H H
│ │ │ │
R – CH2 - C∙ +
R- CH2 – C. à R – CH
= C + H– C – CH2 – R
│
│
│ │
Cl Cl Cl Cl
The products are known as dead polymers.
TOPIC – 2 - PLASTICS
Definition: Plastics are high polymers which can be
moulded into any desired shape under proper conditions of temperature ,
pressure and catalyst. (e.g) PVC , PET
Advantages of
plastics:
Disadvantages of low quality
plastics:
1. Insulator 1. very soft
2. Corrosion resistant 2.
Embrittlement
3. Easy mouldability 3. Agening ( Low durability)
4. Used as shock absorbers 4.
Cannot withstand high temperatures.
5. Has adhesive property 5.
Creep (shape Deformation due to load)
6. Less weight
7. Chemical inertness
8. Available in
various colours
CLASSIFICATION OF PLASTICS:
a)Based on thermal properties - i) Thermo plastics ii)Thermo setting plastics
b)Based on utility
- i) Commodity plastics ii) Engineering plastics
Differences between Thermoplastics and thermosetting
plastics
No
|
THERMOPLASTICS
|
THERMOSETTING PLASTIC
|
1
|
Eg. PVC , Polyethylene
|
Polyester, Bakelite
|
2
|
Plastics which are melted at high temperature, solidified
at low temperature They can be remelted
and remoulded into any desired shapes for any number of times.
|
They cannot be remoulded after their first usage.
|
3
|
Scarp can be used again.
|
Scarp can not be used again.
|
4
|
Formed by addition polymerisation
|
Formed by condensation polymerisation
|
5
|
They have linear structure
M – M – M – M – M – M
|
They have complex 3D structure.
- M -
M - M
- M –M
│ │
│ │ │
M -M
- M -
M - M
│ │
│ │ │
M -
M - M – M – M
│ │
│ │ │
|
6
|
The bond strength is low
|
The bond strength is high
|
7
|
Molecular weight is low
|
Molecular weight is high
|
8
|
Soluble in organic solvents.
|
Insoluble in organic solvents.
|
9
|
Prepared by Injection moulding
|
Prepared by compression moulding.
|
Differences between Commodity and Engineering plastics
No
|
COMMODITY PLASTICS
|
ENGINEERING PLASTIC
|
1
|
Eg. Low grade PVC ,polystyrene, Polyethylene
|
PVC, Teflon , Polycarbonate, poly urethane, Nylon, PET
|
2
|
Used for domestic and general purposes.
|
Used for special
and engineering purposes
|
3
|
Easily affected by chemicals
|
Not affected by
most of the chemicals.
|
4
|
Thermal property is very poor.
|
Thermal property is verygood.
|
5
|
They are not 100 % insulators.
|
They are having
high insulating properties.
|
6
|
They cannot withstand abrasion.
|
They can withstand abrasion.
|
7
|
Mechanical strength is low.
|
Mechanical strength is high.
|
8
|
Comparatively cheap.
|
Comparatively costly.
|
PROPERTIES AND USES OF ENGINEERING
PLASTICS
No
|
Name
|
Properties
|
Uses
|
1
|
PVC
|
1.Colourless , odourless powder.
2. Affected by Organic
chlorinated acids.
3. It is degraded by high
temperature and radiations.
|
1.Pipes
2Electrical wire covering
3.Table cloth
4.Adhesives
|
2
|
TEFLON
|
1. Except Fluorine, it is
chemically inert.
2.Withstands up to 3500C
3. Electrical insulators.
|
1. In chemical carrying pipes
2.Gaskets in cookers
3. Electrical switchboards.
|
3
|
POLY
CARBONATE
|
1. Withstand very high
temperatures.
2. They are having high
transparency.
|
1. In sterilizable bottles.
2. Film industry – Camera,
Photography films
3. Transparent bottles
|
4
|
POLY URETHANE
|
Used at Subzero (-ve)
temperatures.
|
1.Oceanography
2.In defense
3.High altitude mountains
|
5
|
PET
|
1.High stretch resistance
2. High wrinkle resistance
3. Unbreakable
4. Acid proof
|
1. PET jars, bottles
2.Helmets
3.Terylene fabrics
4.Textile , wool industry
|
6
|
POLY AMIDES
|
1.Flexibililty
2.Elasticity
3.Elongatable property
|
1. Tooth brush bristles
2.Automobile gears
3.Textile industry
4. Nylon ropes
|
PREPARATION OF SOME IMPORTANT ENGINEERING PLASTICS
1. PVC – POLY VINYL
CHLORIDE
Step 1 –
Acetylene is treated with Hydrochloric acid at 60-800C in presence
of some metallic chloride catalyst. It forms Vinyl chloride.
CH≡CH + HCl
MCl / 60-800C CH2 = CH
Cl
(Acetylene) (Vinyl
chloride)
Step 2 – Vinyl
chloride, in presence of Hydrogen peroxide undergoes polymerisation to form
Poly vinyl Chloride.
 |
n 

 CH2 = CH H2O2 CH2 CH
CH2 = CH H2O2 CH2 CH
Cl Polymerisation
Cl n
Vinyl Chloride PVC
2. TEFLON (Poly Tetra
Flouro Ethylene –PTFE)
Step – 1 When Chloro
Diflouro methane is heated, it forms Tetra flouro ethylene is formed.
2CHClF2  heating CF2 = CF2
heating CF2 = CF2
(Chloro difluro methane) (Tetra flouro ethylene)
Step 2 - Tetra flouro ethylene , in presence of Benzoyl peroxide (Be2O2)
, polymerized to form PTFE.
polymerisation
(Tetra flouro ethylene) (Teflon – PTFE)
3. POLY CARBONATE ( Lexan /
Merlan)
_O
CH3
n C =
O +
n OH - __C __ --OH
à
CH3
O CH3
║ l
-- (O—C—C6H4—C-- C6H4—O)n-- +2n
C6H5OH
l
CH3
4.POLY URETHANE ( Perlon – u)
O O
║ ║
nC = N – (CH2)6
– N = C + n
HO – (CH2)4 – OH à
(Hexa methylene di iso
cyanate) (Butane diol)
O
O
║
║
(-C
– NH – (CH2)6 – NH – C – O – (CH2)4
– O- )n
(poly
urethane)
5. POLY ETHYLENE TERYPTHALATE
(PET)
n HO – (CH2)2 - OH
+ n HOOC - - COOH
à
Ethylene
glycol Terephthalic
acid
(-O – (CH2)2
– O – C - - C - --C--)n + (2n-1)
H2O
║
║
O O
(PET)
6. POLYAMIDES ( NYLON -6,6)
Condensation Polymerisation
n H2N - (CH2)6 – NH2 + n HOOC
– (CH2)4 – COOH à
Hexa methylene diamine Adipic
acid
[ - HN - (CH2)6 – NH - OC –
(CH2)4 – CO - ]n + (2n-1)H2O
Nylon 6,6
TOPIC – 3 - RUBBERS
Rubber is a high polymer with basic qualities of elasticity and
non-crystallinity. They are known as elastic + polymer = elastomers.
Types – i) Raw (natural) rubber ii) Synthetic rubber
Natural rubber preparation:
1. When we cut the bark of rubber
tree, latex milk is coming out. It is
collected in containers.
2. Latex contain major portion of
water (70%) and 30% rubber as isoprene C5H8 units.
3. To get colloidal rubber, as
coagulant, we are adding acetic acid.
4. The colloidal rubber is dried
in air or passing smoke. The previous
one is called ‘dried rubber’ and another one is ‘smoked rubber’.
5. Then it is made as sheets using
rollers.
Vulcanisation:
To remove the defects of rubber,
we are adding sulphur to rubber and heating at 100 – 1400C under
high pressure. This process is called
Vulcanisation.
The defect of rubber is mainly due to the
non-cross linked isoprene structure of rubber.
But, the added sulphur attacks the double bond of isoprene units and
converts the non-cross linked structure
into a cross linked structure. So, the defects of natural rubber are removed.
If 3 -5 % sulphur is added, it is soft
rubber. They are used in tyres.
If more than 30% Sulphur is added, it is
called ‘hard’ or ‘ebonite rubber’. They are used in acid battery cases.
Isoprene
C5H8 à Poly isoprene
CH3 CH3 CH3
│ │ │
CH2 = C – CH = CH2 à –CH2 – C= CH–CH2 –CH2 – C = CH – CH2 –
–CH2 – C= CH–CH2 –CH2 – C = CH – CH2 –
│
│
CH3 CH3
( Rubber defects due to non-cross linked structure)
Adding sulphur during
vulcanization alters the structure as follows:
CH3 CH3
│ │
–CH2 – C– CH–CH2 –CH2 – C – CH – CH2 –
│ │ │ │
S S S S
│ │ │
│
–CH2 – C– CH–CH2 –CH2 – C – CH
– CH2 –
│ │
CH3 CH3
Differences between raw and vulcanized rubber
No
|
Raw rubber
|
Vulcanised
rubber
|
1
|
Soft and sticky during summer
|
Not Soft and sticky during
summer
|
2
|
Hard and brittle during winter
|
Not Hard and brittle during
winter
|
3
|
Swells in oil
|
Does not Swell in oil
|
4
|
Absorbs high amount of water
|
Does not Absorb high amount of
water
|
5
|
Affected by organic and
inorganic acids
|
Not Affected by organic and inorganic acids
|
6
|
Easily undergoes oxidation
|
Does not easily undergo
oxidation
|
7
|
Poor life time
|
High life time
|
8
|
Tensile strength is low (200
kg/cm2)
|
Tensile strength is high (2000
kg/cm2)
|
9
|
Used between 10 – 60 0C
|
Used between - 40
to 100 0C
|
10
|
E.g) Raw rubber
|
E.g) SBR , Butyl rubber
|
SOME IMPORTANT SYNTHETIC RUBBER
1.SBR – Styrene Butadiene
Rubber – Buna -S
n CH2 = CH - CH
= CH2 + n à
( 75% butadiene)
(25% Styrene)
[
CH2 - CH = CH
- CH2 - CH2 -- CH
-]n
(Styrene
– Butadiene RubberSBR)
properties:
1. For vulcanization, it needs
little amount of sulphur but more amount of accelerators.
2. High tensile strength.
3. Not Soft and sticky during
summer
4. Not Hard and brittle during
winter
5. Not affected by organic and
inorganic acids.
Uses:
Tyres – Belts – Gaskets – Shoes –
Tank linings
2. BUTYL RUBBER – (GR-I)
rubber
CH3
│
n [ H2C = C (CH3)2
] +
n [CH2 = C – CH = CH2] à
CH3
│
Properties:
1. Low permeability to air 2. High
tensile strength. 3. Not Soft and sticky during summer 4. Not Hard and brittle
during winter 5. Not affected by organic and inorganic acids.
Uses:
Inner tubes of automobile tyres -
Belts – Gaskets – Shoes – Tank linings
TOPIC 4 – COMPOSITES AND FIBRE REINFORCED
PLASTICS (FRP)
COMPOSITES
# Composites are the special kind
of material system consist of two distinct phases – matrix and dispersed phase.
# Matrix phase is the external
continuous body constituent.
(e.g) Ceramics, Polymer, metals
# Dispersed Phase is the internal
structural constituent.
(e.g) Fibre, Flakes, particulates
Need / Advantages / Properties of composites:
1. They are inert towards
chemicals.
2. Corrosion resistance
3. Improved mechanical strength.
4. Improved creep resistance.
5. High insulation property.
6. Variable dielectric constant.
7. Withstand very high
temperature.
8. High dimension stability.
Types of composites (Based on Matrix)
1. Polymer matrix composite(PMC)
2.Metal matrix composite (MMC)
3. Ceramic matrix composite(CMC)
POLYMER
MATRIX COMPOSITE ( FIBRE REINFORCED PLASTICS –FRP)
Synthesis:
Step 1.
Polymer plastic resin is taken as
matrix phase.
Example:
S.No
|
Resin
|
To provide
|
1
|
Polyester
|
Good strength and mechanical
property
|
2
|
Epoxy resin
|
Tensile and mechanical strength
|
3
|
Silicone resin
|
Excellent thermal and electrical
property
|
4
|
Phenolic resin
|
High temperature resistance
|
5
|
Aromatic amide ( Aramid) resin
|
Reusable nature
|
Step 2.
Fibre is taken as Dispersed Phase.
Example:
S.No
|
Fibre
|
To provide
|
1
|
Alumina
|
Dimension stability
|
2
|
Boron
|
Stiffness
|
3
|
Glass
|
Corrosion resistance
|
4
|
Carbon
|
Bio compatibility
|
5
|
Graphite
|
Lubrication
|
Step 3.
Matrix Phase + Dispersed Phase are suitably
mixed, and cured under proper heat and pressure. This results in FRP.
COMMON APPLICATIONS OF FRP:
1. Medical field
2. Aero planes
3. Air crafts
4. Automobiles
5. Sports
6. Industries
7. Submarines
8. Pollution control
1. Medical field: - For hip joints and fracture curing plate
treatment, steel may have some side effects due to non-bio compatibility. But Carbon FRP plates are highly bio
compatible and they are used as plates.
2. Aeroplane: - In Aero planes vertical and horizontal wings,
Boron FRP are used due to their very low mass / volume ratio. It is 30% lighter than steel.
3. Air crafts: - In aircraft wings, high resistant blocks, nose
cones of missiles, Carbon or graphite FRP are used.
4. Automobiles -Due to high
mechanical strength, they are used in automobile parts.
5. Sports - Due to high water resistivity, graphite, silicone FRPs are
used in sports mats and artificial sports lawns.
6. Industries - As they are not affected by chemicals, they
are used in chemical industries as reservoirs.
7. Submarines - Due to high water resistivity and
strength, Glass FRP and silicone FRP are used in submarines.
8. Pollution control - As they are easily bio degradable, their
pollution problem is minimum.
SPECIFIC EXAMPLE:
CARBON FRP:
No
|
TYPE
|
PROPERTY
|
USES
|
1
|
Carbon- Polyester FRP
|
1.Bio compatability
2.Improved mechanical property
|
1. Medicine
2. High load automobiles
|
2
|
Carbon – Epoxy resin FRP
|
1.Good tensile and
mechanical strength
|
Chemical industries
|
3
|
Carbon – Phenolic FRP
|
1.High temperature resistance
2.Friction resistance
|
1.Aircraft,
2. Aeroplanes
|
4
|
Carbon – Silicone FRP
|
1. Bio compatability
2. Excellent thermal and electrical nature
|
1. Sports lawns
2. Submarines
3. Medicine
|
5
|
Carbon – Aramid FRP
|
1. Bio compatability
2. Reusability
|
1. Pollution free plastics
|
In Similar way , we can have glass FRP, Alumina FRP, Boron
FRP etc.,.
No comments:
Post a Comment